It is taken that you have read our Disclaimer and Privacy Policy on entering this website
"Everyone has the right to freedom of opinion and expression; this right includes freedom to hold opinions without interference and to seek, receive and impart information and ideas through any media and regardless of frontiers"
Article 19 UN Declaration of Human Rights to which Australia is a signatory
Natural Therapies Products and Supplements under threat from Agenda 21 dictate
Inappropriately Botched
 |
by Lynda Brownsey Sep 2011 |
There definitely do exist drugs and practices out there which require the attention, prompt investigation and action by the relevant authorities and full exposure in the media (if we had a free media).
Unfortunately, these tasks are neglected for Agenda 21 Mischief. It would seem to be more important to pass legislation banning and criminalizing vitamins and the kitchen herb garden and proscuting 'an apple a day keeps the doctor away' health claims. Harassment of the practice of traditional medicine would be a given.
These political agendas are more usually than not, related to powerful pharmaceutical interests which would have the effect of restricting choices to natural alternatives to prescription meds. Such is Agenda 21 Mischief.
And this is your tax dollars at work, Australia.
Therefore, this webpage is dedicated to the growing number of real victims whose legitimate claims and reports should exercise the minds, consciences and resources of a real government and real media serving the interests of all the people. (Shoot me, I'm an idealist). For example, real victims of the drug Aldara (generic Imiquimod) have been ignored for over a decade and fobbed off by the medical profession and regulatory bodies.
For information on adverse reactions experienced by some users of Aldara watch Elaine Hollingsworth's videos on www.doctorsaredangerous.com.
The complications of this drug suffered by so many people with the reported number of adverse reactions has now grown to such an extent that a private class action on behalf of Aldara victims (many of whom are Australian) is now being prepared in the United States by the firm Parker, Waichman, Alonso - a national law firm.
This is the true context of
The Great Pan Pharmaceuticals Product Recall of 2003
"When the quality of a medicine can not be certain, neither can the safety or effectiveness of that medicine."
Regulatory action and product recall information:
http://www.tga.gov.au/saftety/recalls-medicine-pan-030428.htm.
April 28, 2003. Sydney, Australia.
 Pan Pharmaceuticals Ltd. (Pan) was raided by the Australian Therapeutic Goods Administration (TGA) on grounds that the company's over the counter pharmacy medicine - Travacalm had included a faulty batch. Although the company had recalled Travacalm voluntarily (two months prior) and was in full compliance with the voluntary recall process, Pan had its license to manufacture suspended by the TGA
Pan Pharmaceuticals Ltd. (Pan) was raided by the Australian Therapeutic Goods Administration (TGA) on grounds that the company's over the counter pharmacy medicine - Travacalm had included a faulty batch. Although the company had recalled Travacalm voluntarily (two months prior) and was in full compliance with the voluntary recall process, Pan had its license to manufacture suspended by the TGA
http://members.iimetro.com.au/~hubbca/pan.htm
Thus began the largest recall in Australian history - not of the pharmacy medicine Travacalm - but of Pan's entire complementary and natural medicine product range. These were subject to the TGA claim that death or permanent injury could occur if they were not urgently recalled. This is a Class 1.
No grounds or evidence ever were provided by the TGA that any of these vitamins, minerals or herbal compounds had caused any deaths or injuries. And the opinion of the TGA was never subject to independent scrutiny because the recalled product was destroyed in toto at a secret location.
Contrast this mischief with the TGA's Class 2 recall of Vioxx which Merck voluntarily withdrew from the market Sept 30, 2004.
http://www.tga.gov.au/recalls/2004/vioxx.htm. This page is now an Error 404: page not found. For Vioxx reports, please see the Merck sections on the Dead Baxter Ferrets Do Not Lie webpage of this website. http://www.redlandbayhomoeopathy.com.au/dead_baxter_ferrets_do_not_lie.php
MD Whistleblower Dr David Graham testified before the US Senate that he estimaged as many as 139,000 Americans had experienced heart attacks as a side effect of Vioxx. http://www.lewrockwell.com/sardi/sardi53.html.
The Wall Street Journal reported on Oct 6, 2004 that Vioxx may have led to more than 27,000 sudden cardiac deaths before Merck and Co pulled it from the market. But Merck's Australian subsidiary, Merck, Sharp and Dohm is still doing business in the Lucky Country.
Not so Pan Pharmaceuticals for which there is not one confirmed death from one of its vitamin, mineral supplements or complementary natural products.
As a practitioner of natural therapies, I recall the events of April 2003 very well. I was taking calls all day and all night from naive persons who were alarmed that their vitamin tablet or their herbal tea might give them a stroke or a heart attack.
The number the TGA ran on Pan ordo ab chao did create total chaos among consumers and retailers.
Once upon a happier time, Pan Pharmaceuticals was the nation's largest manufacturer of complementary medicines - vitamins, minerals, herbal and nutritional supplements. It manufactured its own line of medicines and it was the contracted manufacturer for prescription based drugs as well as for 80% of all alternative medicines for providers across Australia. It specialized in the manufacture of natural supplements for other brand names.
According to the TGA website, they suspended Pan's license after an audit of Pan's manufacturing premises. The TGA inspectors found "deficiencies and failures in the manufacturing quality control procedures." Thus, the TGA recalled all batches of medicines manufactured by Pan supplied to the Australian market since May 1, 2002. In also cancelled 1650 products for export made by Pan.
On June 23, 2003, Pan was placed into voluntary liquidation.
Also by June, the TGA hadded dozens more products to the Recall - 100 vitamin products and herbal medicines: in all 219 products.
The companies which held proprietary rights to the recalled products were slapped with a deadline to disclose to the TGA the complete list of their products and batches that had been made by Pan.
The TGA found Pan was complicit in the substitution of ingredients, falsification of test results and substandard manufacturing processes.
Kirsty Needham AAP. The Age. June 3, 2003 "Pan Recall Blows Out to 1624 Products."http://www.theage.com.au/articles/2003/06/03/1054406141529.html
The Western Australian Commissioner for Consumer Protection advised consumers who had products subject to the Recall to return them to the place of purchase for a full refund. So the companies whose products were manufactured by Pan had to shoulder the cost of the refunds.http://www.commerce.wa.gov.au/Corporate/Media/statements/2003/April/PAN_Pharmaceuticals_.html
These companies were also ordered by the TGA to meet the costs of their own recall advertising.
On the very day of the TGA Recall, the Fix Was In and the Spin was Spinning. Here's the Beat-Up by the media.
"Pan Pharmaceuticals Recall" PM Monday 28, April, 2003
http://www.abc.net.au/pm/content/2003/s842036.htm
Mark Colvin: "But first tonight, don't take your vitamins and check your other non-prescription drugs. Australian medicine cabinets tonight need a severe clean-out."
Follow carefully, readers, as Mark Colvin tells us in the succeeding remarks how Pan's manufacturing premises (found by the TGA audit to be in such breech and subject to such failure as to warrant a Class 1) was up to speed with the manufacture of its prescription meds.
John McEwen, the TGA's principle medical advisor, allayed public fears regarding the meds manufactured by Pan for the PBS. These medicines are fine, he reassurered tv land. Not so the entire range of natural and complementary medicines. Those were suspect. "What we are talking about are a range of herbal and vitamin products and similar products to that."
So. Those prescription drugs produced by Pan were still on the shelves on the night of 28 April 2003 and given a pass by John McEwen in despite that they were produced in the same mixing machines as the banned natural health products subject to the Class 1 Recall.
In this interview, the ABC made quite the effort to keep those bad vitamins, supplements and natural medicines distinct in the public mind from the good pharmaceuticals that Pan was also contracted to manufacture.
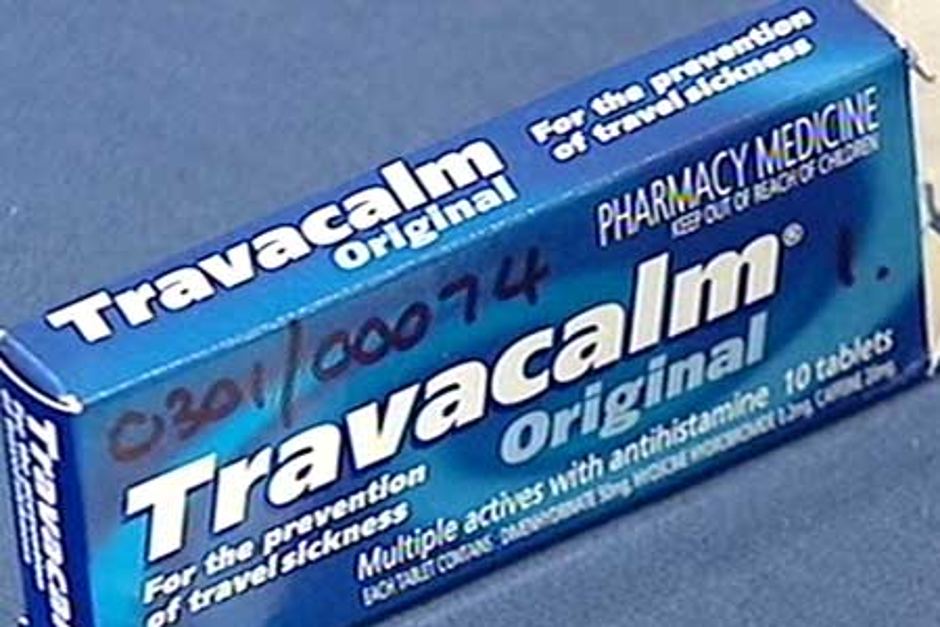 Nick Grimm: "The product recall is the result of an investigation which began in January when an anti-travel sickness tablet, Travacalm, manufactured by Pan for another company, was itself recalled. Faulty batches of the product had caused the hospitalisation of 19 people, while 68 others sufferred potentially life-threatening reactions."
Nick Grimm: "The product recall is the result of an investigation which began in January when an anti-travel sickness tablet, Travacalm, manufactured by Pan for another company, was itself recalled. Faulty batches of the product had caused the hospitalisation of 19 people, while 68 others sufferred potentially life-threatening reactions."
Just try to find any report of this prior to April 28, 2003. I'm still looking. But in August 2005, the Supreme Court of Victoria approved a Settlement Agreement which provided for the assessment of group member claims and for payment of damages. Settlement monies were paid in 2006.
So. what were those hospitialisations and thos potentially life-threatening adverse reactions? The webpage linking to those 87 Travacalm reactions on www.tga.gov.au/recalls/2003/panqac.htm reads Error 404: page not found.
The bottom line of assurance dispensed by the ABC was: "It's important that we stress that we're not talking about prescription medications. Up to this time, no safety problems have been identified with the prescription medicines on the Public Benefits Scheme.
"What we're talking about are a range of over-the-counter and particularly complementary medicines."
Yes. TV Land - it's that parsely, sage, rosemary and thyme that will do you in if the TGA wasn't on the job.
By Day 3 another 449 products had been recalled and the Debacle was bigger than Ben Hur.
The 7:30 Report located a Pan machinist who was willing to 'put his job on the line' and reveal the culture of bullying and incompetence at Pan's manufacturing plant. The macinist was one Richardson Obenoza who got his 5 mins of fame whining that the CEO of Pan, Jim Selim, threw temper tantrums and that "you have to make 5 million tablets a day, but we can't make that if we have to clean the machine properly."
http://www.abc.net.au/7.30/content/2003/s844037.htm
For ABC coverage of 'the Pan Debacle' see
http://search.abc.net.au/search/search.cgi?form=simple&num_ranks=20&collection=abcall&query=PAN+Pharmaceuticals+Recall
This was not good.
After days of fishing and no complaints against class 1 recall products had surfaced from the public. The story began to fizz. It was beginning to look like exactly what it was - the TGA had run a number on Pan. For all the media attention, the largest recall in the history of the nation was getting, the bodies should have been piled in the streets. Instead people were getting bored. Temper tantrums and quotas were not going to cut it on prime time. What to do? The reasons for closing down Pan had to shift to something that would suit the public disinterest in the fizzing story - something more technical. In the end, it had to be some inscrutable offense that only the experts could determine and argue about.
 So they settled on defective manufacturing processes.
So they settled on defective manufacturing processes.
By June 2003, the TGA added dozens more Pan products to the class 1 recall - 100 ranges of vitamin and herbal medicine lines.
Companies which held proprietary rights to some of these products were slapped with a deadline to disclose to the TGA all the products and batches manufactured by Pan. These companies were ordered by the TGA to meet the costs of their own recall advertising. The TGA then found that Pan was complicit in the substitution of ingredients, falsification of test results and substandard manufacturing processes.
And for this, the TGA was set to keep Mr Jim Selim and his board of directors very busy in court. The CEO of Pan Pharmaceuticals, Jim Selim would be facing criminal charges. And this went on for five years.
Pan was placed in the hands of the Swiss liquidators in 2005 and gutted. KPMG are Big Machers who specializein mergers, liquidations, chemicals and pharmaceuticals.
Now, this demonization of complimentary medicines by the TGA would not be entirely objectionable, if it were not that victims of a pharmaceutical like Aldara (unleashed by 3M Pharmaceuticals in 2000) still await some form of official interest-let alone justice.
In August 2008, Jim Selim won a $55 million compensation from the Commonwealth government which 'inappropriately' recalled Pan's entire 1600 product suite in 2003 and liquidated his company.
The Pan Story was now back in the news. Selim announced that together with shareholders and companies affected by the TGA recall, he would go ahead with preparations for a 200 million class action against the TGA over the collapse of Pan in 2003.
One hundred companies lined up to sue the Commonwealth and two class actions formed up. The first was on behalf of Pan's shareholders and the second was on behalf of companies who had contracted with Pan to manufacture their products.
 Then, the criminal prosecution against the company and Selim was quietly dropped Sept 19, 2008.
Then, the criminal prosecution against the company and Selim was quietly dropped Sept 19, 2008.
On March 26, 2011, The Sydney Morning Herald reported that the cost of the Federal government's "botched handling" of the now liquidated Pan Pharmaceuticals had topped $122.5 million.
The media mindwarp here is the presumption that if the TGA had handled their mischief mischief making and Twat Control in a correct manner, the government would not have been liable. But liable they were.
When the dust finally settled on the class action, the government was up for a further $67.5 million.
This is your tax dollars at work Australia.
The Federal Court Justice Geoffrey Flick approved additional compensation to be paid to a group of the company's 162 creditors, distributors and retailers who sufferred loss and damage when the TGA 'inappropriately' closed down Pan in 2003.
This settlement brought to a close the embattled Australian company's 8 year ordeal that began with the TGA suspension of Pan's license and class 1 recall of Pan's entire range of natural and complimentary products - the largest in the nation's history. This action by the TGA cost 1000 jobs and up to 450 million in lost revenue to the suppliers, pharmacies, health food shops and other retailers. "Pan Debacle Yields Further $67.5 Million Payout" Louise Hall. The Syndey Morning Herald. Marych 26, 2011.
Invoking the 'watchword' of the Cryptocracy, Andrew Thorper, a partner at Mclachlan Thorpe Partners and litigation funders IMF Australia stated for the record "I think it is fair to say that the industry descended into a state of chaos from which it has never fully recovered".
And this was, of course, the point of the entire exercise.
Agenda 21 ordo will emerge from the chao - as in ordo ab chao.
And that too is the point of the entire exercise.

under construction